
Pharmaceutical Risk Management Safety: Enhancing Production Standards
In the fast-paced pharmaceutical industry, maintaining safety and managing risks are paramount. Innovations in risk assessment, worker safety, and technological advancements have transformed how pharmaceutical companies operate. This blog explores new methodologies for risk assessment and mitigation, innovations in worker safety, and we’ll discuss practical solutions like laboratory dividers and clean room solutions that further enhance safety and compliance.
Advanced Risk Assessment Tools
Effective risk management begins with comprehensive risk assessment tools. Here are some advanced methodologies making a significant impact:
1. Failure Mode and Effects Analysis (FMEA):
FMEA systematically identifies potential failure points in the manufacturing process. By assessing the severity, occurrence, and detection of each potential failure, companies can prioritize risks and implement targeted mitigation strategies, reducing the likelihood of critical failures.
2. Hazard Analysis and Critical Control Points (HACCP):
Originally developed for the food industry, HACCP is now widely used in pharmaceuticals. It involves identifying, evaluating, and controlling hazards that could compromise product safety, ensuring that every stage of production meets stringent safety standards.
3. Bow-Tie Analysis:
Bow-Tie Analysis combines qualitative and quantitative risk assessment by visualizing pathways from potential causes to consequences. This method highlights preventive and mitigative measures, providing a clear framework for managing risks.
Integrated Risk Management Systems (IRMS)
To effectively manage risks, pharmaceutical companies are adopting Integrated Risk Management Systems (IRMS):
Holistic Risk Management:
IRMS integrates risk management processes across all departments, from research and development to production and distribution. This system allows for real-time risk assessment and management, fostering a proactive approach to safety and compliance.
Software Solutions:
Advanced IRMS software tracks and analyzes risk data, automates risk assessment processes, and provides real-time reporting. These tools ensure that all levels of the organization are aware of potential risks and mitigation efforts, enhancing overall operational efficiency and safety.
Predictive Risk Modeling
Predictive risk modeling uses data and technology to anticipate and mitigate risks:
- Big Data and Predictive Analytics
Leveraging large datasets and machine learning algorithms, predictive analytics optimize supply chain management and production schedules. These tools identify patterns indicating a higher likelihood of equipment failure, contamination, or other risks, allowing preemptive measures to be taken.
- Simulation and Scenario Analysis
Virtual simulations of the manufacturing process help identify potential risks and test mitigation strategies without disrupting actual production. Scenario analysis allows companies to explore different outcomes and prepare for various risk scenarios, enhancing readiness and resilience.
Innovations in Worker Safety
Worker safety is a top priority in pharmaceutical production. Innovations in ergonomic improvements and protective equipment are crucial:
Ergonomic Improvements
- Ergonomic Workstations:
Designing workstations to reduce physical strain and improve comfort is essential. Adjustable benches, anti-fatigue mats, and ergonomically designed tools minimize the risk of musculoskeletal disorders.
- Exoskeletons:
Wearable devices, such as exoskeletons, support and enhance worker movements, reducing strain and preventing injuries. These are particularly useful in tasks involving repetitive motions or heavy lifting.
- Training and Awareness Programs:
Regular training on ergonomic practices and the importance of maintaining good posture is vital. Employees should be encouraged to take breaks and perform stretching exercises to prevent strain.
Advanced Protective Equipment
- Smart Personal Protective Equipment (PPE):
The integration of sensors and IoT technology into PPE is revolutionizing worker safety. Smart helmets can detect exposure to harmful gases, while smart gloves provide feedback on proper hand movements.
- Enhanced Respiratory Protection:
Innovations in respirator design, such as powered air-purifying respirators (PAPRs), provide cleaner air and reduce breathing resistance, improving comfort and safety for workers in environments with airborne contaminants.
- Impact-Resistant Clothing:
Developments in materials have led to better protection against cuts, punctures, and impacts. This clothing offers enhanced safety without compromising comfort and flexibility.
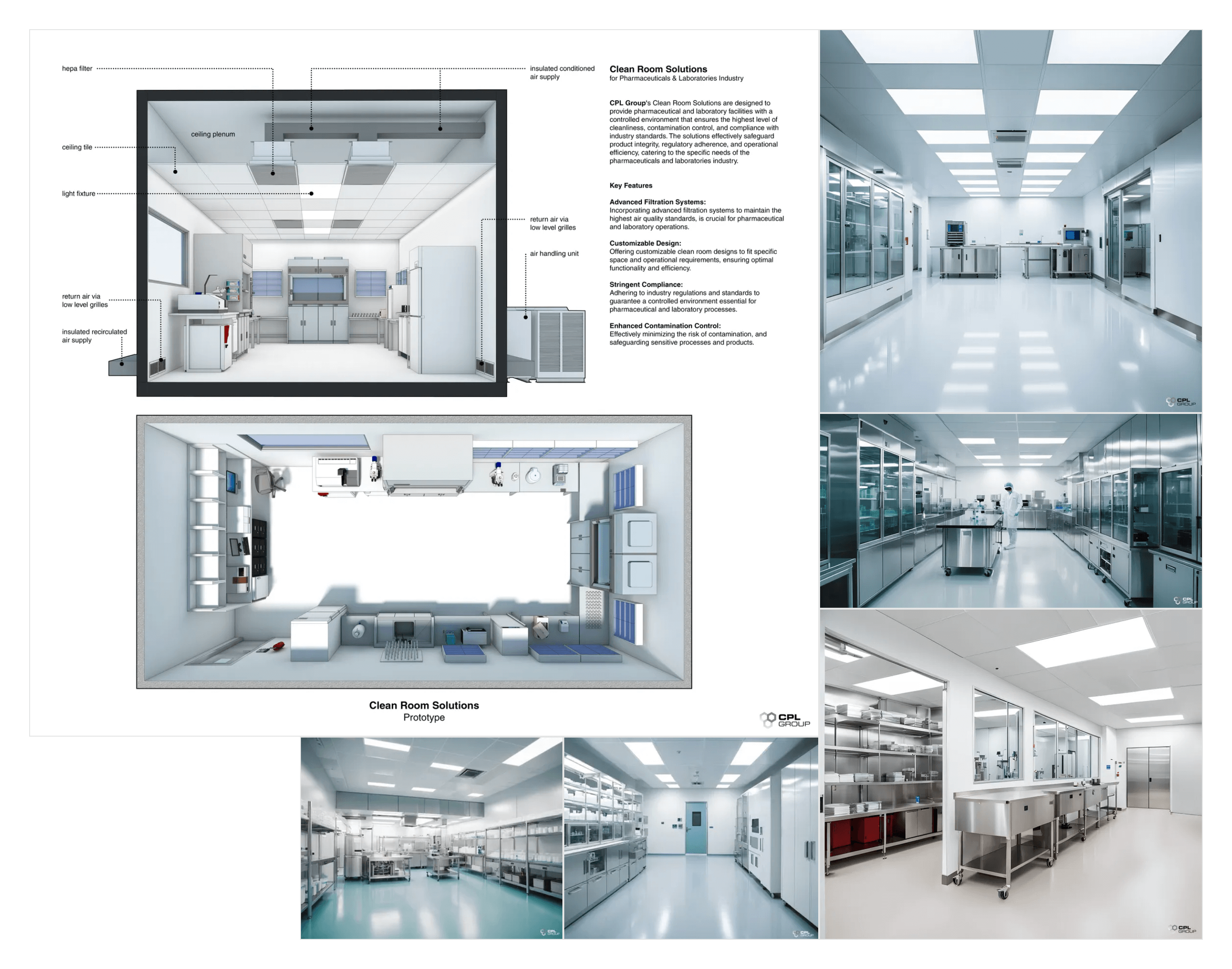
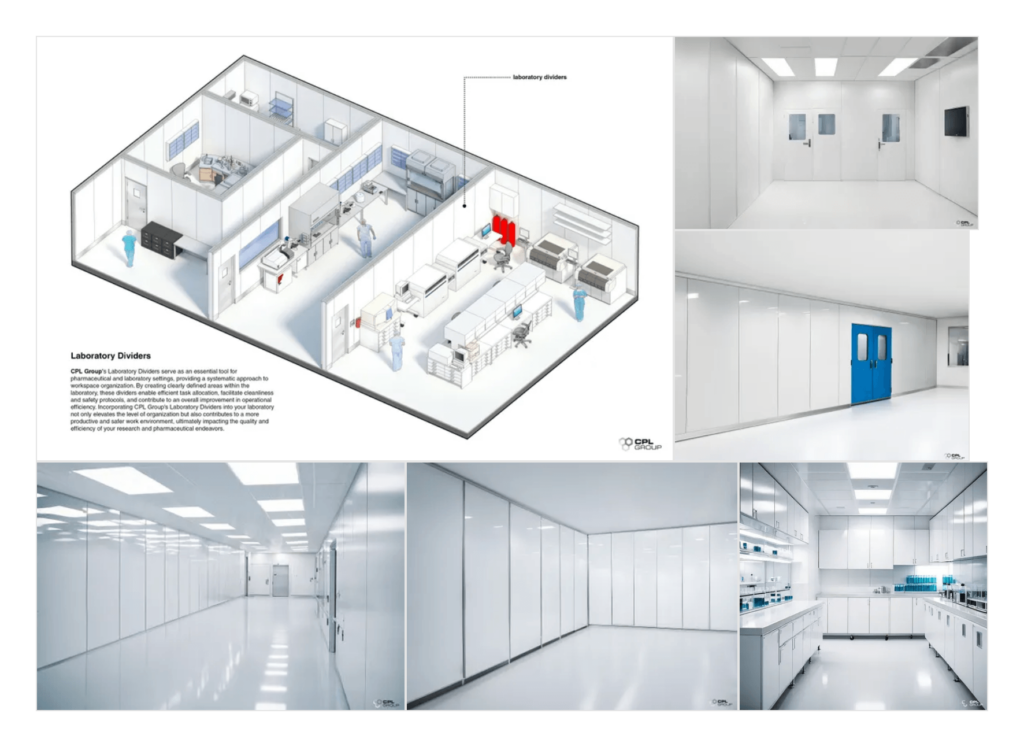
Practical Solutions: Laboratory Dividers and Clean Room Solutions
In addition to advanced technologies and methodologies, practical solutions like laboratory dividers and clean room solutions are essential for maintaining a safe and compliant pharmaceutical production environment:
Laboratory dividers create distinct workspaces within a laboratory, reducing cross-contamination and ensuring a controlled environment for different tasks. These dividers enhance safety by preventing the spread of hazardous substances and ensuring that each workspace meets specific safety requirements.
Clean rooms provide a controlled environment with low levels of pollutants. Advanced clean room solutions, such as HEPA filtration systems and air showers, maintain the required cleanliness levels. These solutions are essential for manufacturing high-quality pharmaceutical products and ensuring compliance with stringent regulatory standards.
Key Takeaways: Why Risk Management is crucial in the Pharmaceutical Industry
Enhancing risk management and safety in pharmaceutical production involves adopting advanced risk assessment tools, implementing ergonomic improvements, utilizing advanced protective equipment, and leveraging technology for real-time monitoring and predictive maintenance. Practical solutions like laboratory dividers and clean room solutions further ensure a safe and compliant manufacturing environment. By embracing these innovations and solutions, pharmaceutical companies can protect their workers, ensure product quality, and maintain regulatory compliance, ultimately enhancing their operational efficiency and productivity.


