
How CPL Group Enhances Compliance & Safety in Pharmaceutical Labs

In the high-stakes world of pharmaceuticals and advanced laboratory research, compliance and safety are not just buzzwords—they’re the foundation upon which trust, reliability, and scientific progress are built. As regulatory landscapes evolve and safety standards become increasingly stringent, facilities face the ongoing challenge of maintaining compliance while optimising their operations. This is where CPL Group’s innovative solutions come into play, offering a comprehensive approach to elevating pharmaceutical and laboratory standards.
The Regulatory Landscape in Pharmaceuticals and Laboratories
Before delving into CPL Group’s solutions, it’s crucial to understand the complex regulatory environment that pharmaceutical companies and research laboratories navigate daily. Key regulatory bodies and standards include:
- FDA (Food and Drug Administration): Sets stringent guidelines for drug manufacturing and laboratory practices in the United States.
- EMA (European Medicines Agency): Provides regulatory oversight for pharmaceutical products in the European Union.
- ISO (International Organization for Standardization): Establishes global standards for clean room classifications and quality management systems.
- GMP (Good Manufacturing Practice): Outlines principles and procedures to ensure consistent quality in drug production.
The challenges in maintaining compliance with these regulations are multifaceted, involving everything from facility design and equipment maintenance to personnel training and documentation processes.
CPL Group’s Approach to Regulatory Compliance
CPL Group has positioned itself as a leader in compliance solutions by adopting a holistic approach to meeting industry standards:
- Adherence to Industry Standards in Clean Room Solutions: CPL Group’s clean room designs are meticulously engineered to meet ISO classifications, ensuring that air quality, particle counts, and environmental controls align with regulatory requirements.
- Customisable Designs to Meet Specific Regulatory Requirements: Recognising that different regions and industries may have varying compliance needs, CPL Group offers flexible solutions that can be tailored to meet specific regulatory frameworks.
- Documentation and Certification Processes: CPL Group provides comprehensive documentation for all installations, including design specifications, material certifications, and performance test results. This thorough approach simplifies the audit process and helps facilities maintain their compliance status.
Enhancing Safety with CPL Group’s Solutions

Beyond regulatory compliance, safety is paramount in pharmaceutical and laboratory environments. CPL Group’s solutions address safety concerns through:
- Advanced Contamination Control Measures: Implementing sophisticated air filtration systems, airlock technologies, and material transfer protocols to minimize the risk of contamination.
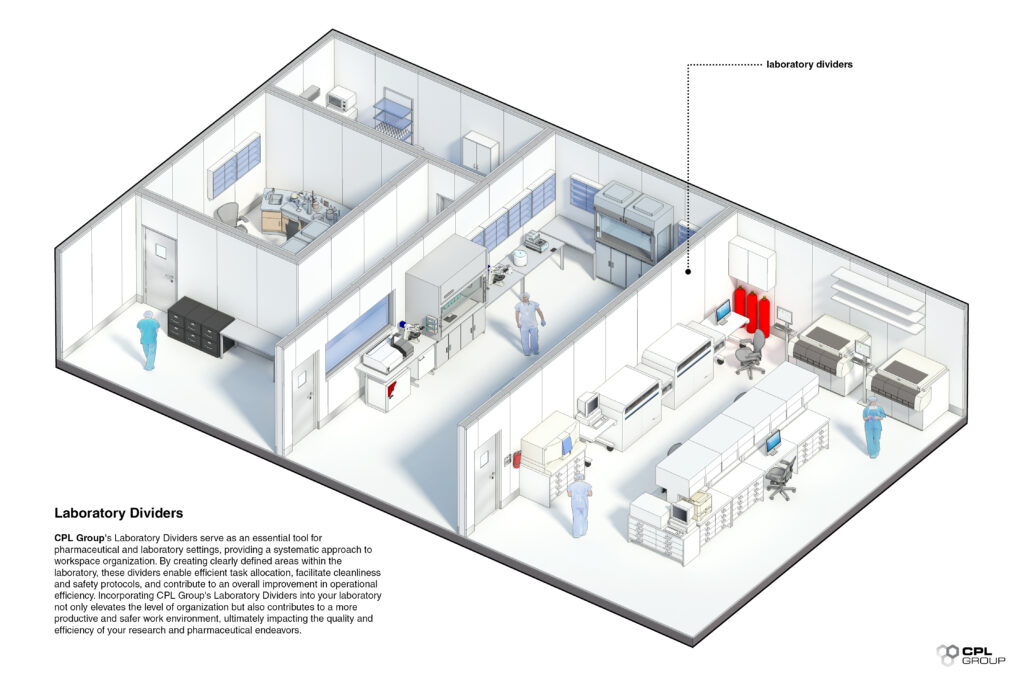
- Laboratory Dividers for Improved Workspace Organisation: Offering modular divider systems that create distinct work areas, reducing the potential for cross-contamination and enhancing overall laboratory safety.
- Material Quality and Durability: Utilising high-grade, chemical-resistant materials in their constructions to ensure long-term safety and reliability in demanding laboratory environments.
The Impact of Compliance and Safety on Business Operations
Investing in compliance and safety solutions from CPL Group yields benefits that extend far beyond merely meeting regulatory requirements:

- Building Trust with Stakeholders: Demonstrating a commitment to compliance and safety enhances credibility with regulators, investors, and the public.
- Improving Product Quality and Research Integrity: By maintaining controlled environments and minimising contamination risks, facilities can ensure more reliable research outcomes and higher-quality pharmaceutical products.
- Reducing Risks and Potential Liabilities: Proactive compliance and safety measures significantly reduce the risk of costly regulatory violations, product recalls, or research setbacks.
Future-Proofing Your Facility with CPL Group
In an industry where regulatory requirements and safety standards are constantly evolving, partnering with CPL Group offers a pathway to future-proofing your facility:
- Staying Ahead of Evolving Regulations: CPL Group’s team of experts stays abreast of regulatory changes, ensuring their solutions always align with the latest industry standards.
- Continuous Improvement and Innovation in Safety Features: Through ongoing research and development, CPL Group continually enhances its product offerings, incorporating new technologies and safety features to address emerging challenges in the field.
Key Takeaways
In the complex and highly regulated world of pharmaceuticals and laboratory research, ensuring compliance and safety is not just about meeting minimum standards—it’s about striving for excellence. CPL Group’s comprehensive solutions offer a clear path to achieving and maintaining this excellence, providing peace of mind in an industry where precision and reliability are paramount.
By choosing CPL Group, you’re not just investing in state-of-the-art clean room technology or laboratory equipment; you’re partnering with a team dedicated to elevating your facility’s compliance and safety standards. This commitment translates into tangible benefits: smoother regulatory processes, enhanced safety records, improved product quality, and ultimately, a stronger position in the competitive landscape of pharmaceuticals and research.
Don’t let compliance and safety concerns hold your facility back. Contact CPL Group today to learn more about how their tailored solutions can transform your approach to regulatory compliance and workplace safety. With CPL Group, you’re not just meeting standards—you’re setting them


